16 Which of the Following Processes Require S Chemical Methods
SDS Section 6 tells you what to do should the chemical be spilled leaked or otherwise released. If 50 g of each reactant were used for the the following process the limiting reactant would be.
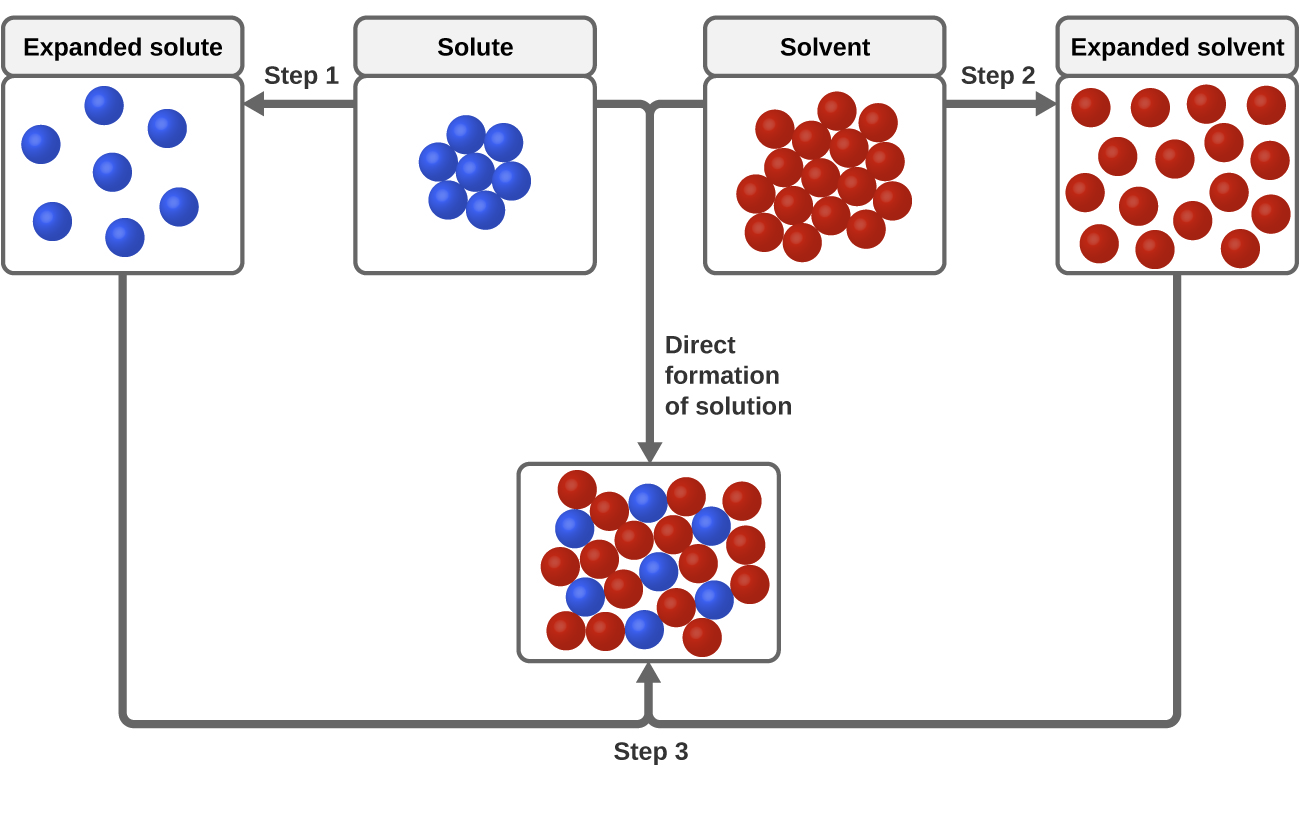
11 1 The Dissolution Process Chemistry
Which process is a chemical reaction.

. Vegetable rotting is a chemical process driven by microorganisms. C has the smallest coefficient. Chemical Process Online Test - Multiple Choice Questions and Answersonline quizonline bitsinterview questions and answers pdf free download for chemical.
The chemical process industries include the following. Freezing of water 3. Baking powder Washing soda Baking soda none of these.
Gasoline evaporating from a gasoline tank 2. Initially the system was comprised of one flask containing matter and another flask containing nothing. It is also known as putrefaction.
Reason Chemical reactions that absorb energy or require energy to proceed are called endothermic reactions. Rusting of a nail 2. E A raindrop falling to the ground.
Instead the driving force appears to be related to the greater more uniform dispersal of matter that results when the gas is allowed to expand. Separating a homogeneous mixture into pure substances B. Copper anode steel object cathode.
Which of the following are chemical processes. The spontaneity of this process is therefore not a consequence of any change in energy that accompanies the process. Reaction with acids from.
In chemical activation processes the precursor is first treated with a. Distilling a saltwater mixture D. Separating a heterogeneous mixture into pure substances C.
When 307 g of Mg reacts with 409 of O2 in the following chemical equation. 2Mg O2 --- 2MgO. A A diagram of the apparatus used for step 1 is shown.
Petroleum refining and petrochemicals Food and beverages Pulp and paper Plastics and polymeric materials Air products oxygen nitrogen etc Consumer products detergents etc Agricultural chemicals Pharmaceuticals Inorganic chemicals sodium hydroxide etc Textiles. The third law of thermodynamics establishes the zero for entropy as that of a perfect pure crystalline solid at 0 K. Which of the following processes is NOT considered to be chemical weatheringA.
The second law of thermodynamics states that a spontaneous process increases the entropy of the universe Suniv 0. D is consumed completely. A Liquid water freezing at a temperature below its freezing point.
Iron rusting when left outdoors 3. Compression of oxygen gas A 2 3 4 B 1 3 4 C 13 D 1 2 E 14. If Δ Suniv 0 the process is nonspontaneous and if Δ Suniv 0 the system is at equilibrium.
Chemical reactions in which heat is released along with the formation of products are called exothermic reactions. Employers are required to adhere to one or more of the following methods in order to determine and evaluate the hazards involved in the process. Δ S univ Δ S sys Δ S surr Δ S sys q surr T.
B Breaking a compound into its constituent elements. Used zeolite is regenerated by flushing with the solution of. A At least two of the above require chemical methods.
Δ S univ Δ S sys Δ S surr Δ S sys q surr T. B has the smallest molar mass formula weight. D A ball thrown into the air.
60 of activated carbon is processed chemically. Zeolite removes both temporary as well as permanent hardness of water by precipitating calcium and magnesium present in water as insoluble zeolites. Exploring Geology with CONNECT Plus 1-semester Access Card 3rd Edition Edit edition Solutions for Chapter 7 Problem 14MCQ.
Process hazard analyses PHAs are designed to identify evaluate and control the hazards of processes involving extremely hazardous chemicals. This is why it can be considered as a chemical reaction. E Separating a homogeneous mixture into pure substances.
Decomposition of water into hydrogen and oxygen gases 4. C Distilling a saltwater mixture D Separating a heterogeneous mixture into pure substances. UCLES 2016 062041MJ16 4 Electroplating steel objects with silver involves a three-step process.
The process is irreversible. Section 7 on your SDS provides a guideline for safely handling and storing chemicals. Which of the following processes requires chemical methods.
Step 3 The coating of silver is applied to the object. Which of the following processes requires chemical methods. In this process the compex organic compounds are broken down by microbes to release nutrients and energy for their growth and development.
E is in excess. Dear Ammar Hamdoon Chemical process is prefered over the thermal. B Liquid water freezing at a temperature above its freezing point.
Chm 1100c ch 5 quizdocx. Required information includes emergency procedures protective equipment and appropriate cleanup and containment methods. At least two of the above a-d require chemical methods.
We may use this equation to predict the spontaneity of a. Carboxymethyl cellulose CMC is added in detergents to. Breaking a compound into its constituent elements E.
Which of the following has sodium bicarbonate as its main constituent. As a result qsurr is a good approximation of qrev and the second law may be stated as the following. C The combustion of gasoline.
Indicate whether the following processes are spontaneous or nonspontaneous. A has the largest molar mass formula weight. Step 2 A coating of nickel is applied to the object.
The limiting reagent in a chemical reaction is one that. Step 1 A coating of copper is applied to the object.

Spontaneous Processes And Chemical Potential Openstax Chemistry 2e 16 1 Youtube

Photosynthesis Cellular Respiration Infographic Photosynthesis And Cellular Respiration Cellular Respiration Photosynthesis
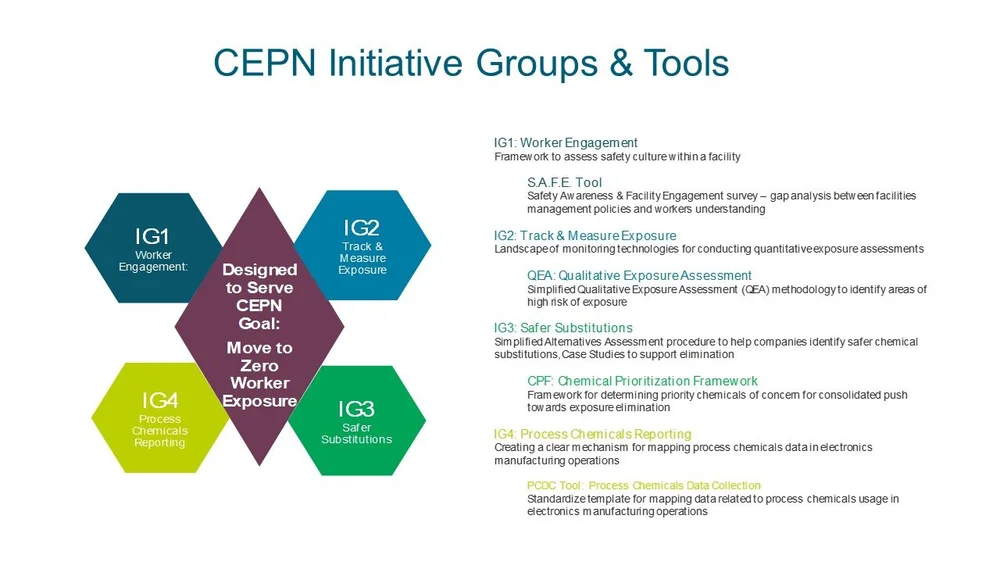
Cepn Main Index Green America Center For Sustainability Solutionstitle

No comments for "16 Which of the Following Processes Require S Chemical Methods"
Post a Comment